No Tissue? No Problem: How a first-of-its-kind blood test optimizes patient management in oncology
By: Joos Berghausen & Dr. Joe Abdo
Category: CiRT
Read Time: 7 Minutes
By: Joos Berghausen & Dr. Joe Abdo
Category: CiRT
Read Time: 7 Minutes
Last updated: 3 June 2024
Discover how a first-of-its-kind blood test is transforming cancer care by predicting immunotherapy success with unparalleled accuracy without the need for invasive tissue biopsies.
Precision oncology gives physicians the ability to personalize therapy strategies for each patient based on the unique molecular profiles that their immune systems and tumors possess. Two commonly used approaches include targeted therapy, where drugs attack specific targets in the tumor, and immunotherapy, where the drug helps the patient's own immune system locate and destroy the cancer.
Over the last decade, there has been tremendous medical advancement in immunotherapy, spearheaded by Immune-Checkpoint Inhibitors (ICIs)1. ICIs target immunologic receptors on the surface of T-lymphocytes (PD-1) or the exterior of the tumor (PD-L1) boosting the immune response to kill cancer cells. Cancer cells bind to the immunological receptors of the T-cells and decrease the host's natural immune response, helping the tumor evade immune surveillance. However, immunotherapy can inhibit the ability of cancer to suppress the host's immune system by binding the immunological receptor on the T-cell first and blocking the interaction between the cancer cells and the T-cell2. Several ICI's utilizing these mechanisms have been FDA-approved and have led to significant clinical benefits and extended survival durations for cancer patients3.
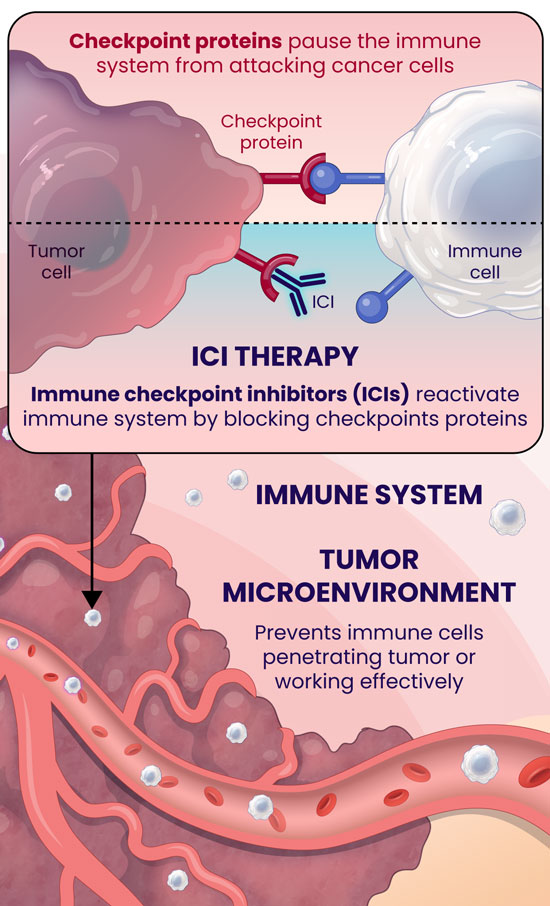
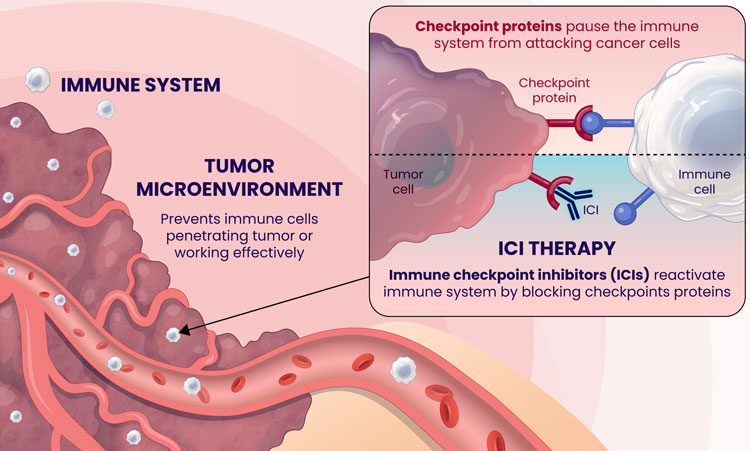
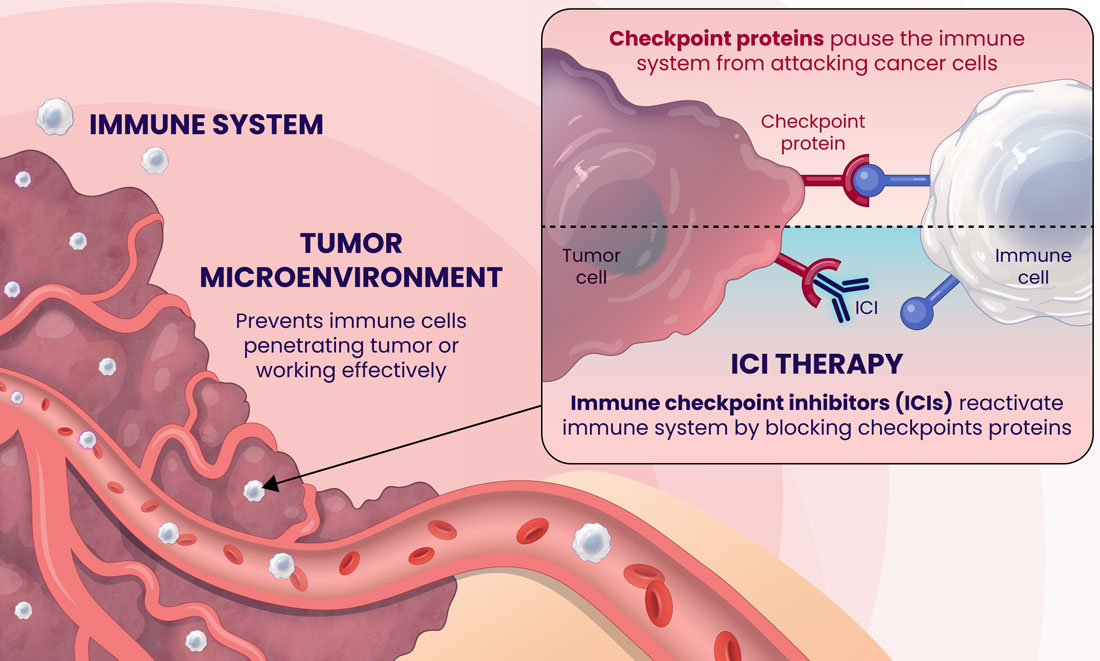
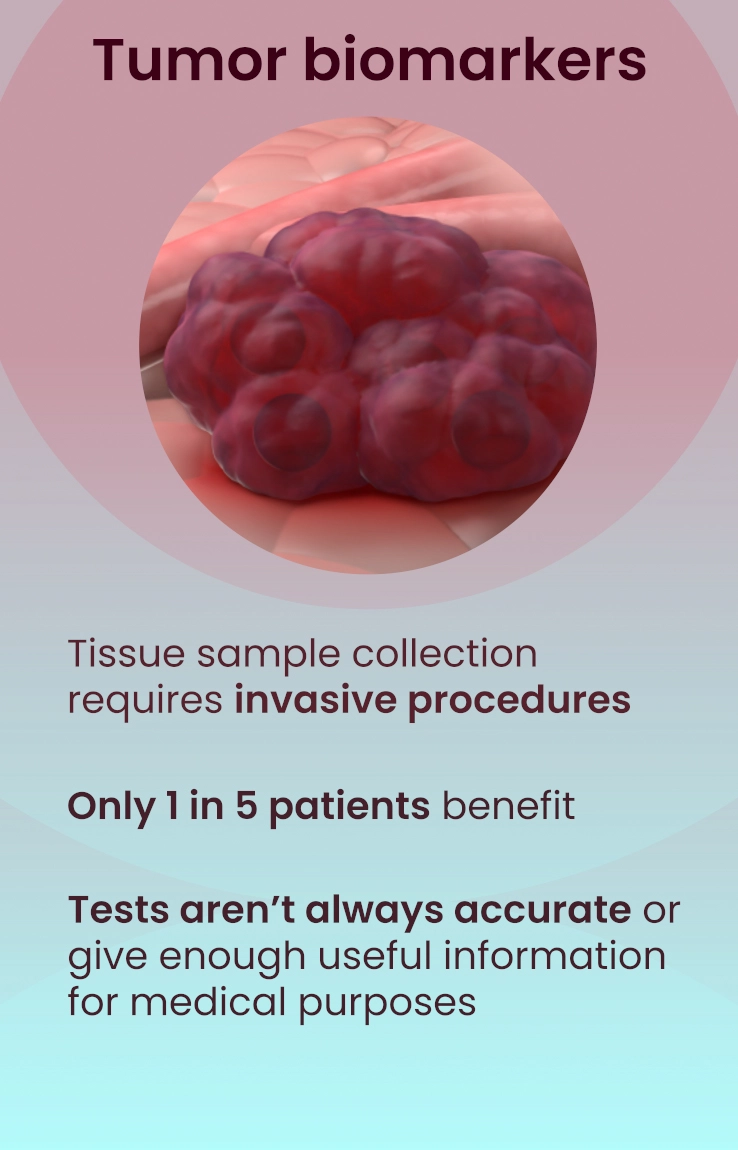
Immunotherapy has revolutionized the field of oncology, offering some patients, albeit a minority, durable improvements. Unfortunately, most cancer patients do not benefit from this treatment, which makes identifying predictive markers for the response to ICIs of utmost importance. The most used predictive biomarker assesses tissue samples from the tumor and determines the expression of PD-L1. However, PD-L1 expression has been well-described in the literature as being an unreliable marker for immunotherapy selection. For example, patients with negative PD-L1 expression can experience clinical benefits from ICIs in dozens of cancers, meaning that thousands of cancer patients who may respond to immunotherapy might not receive it due to the absence of this marker from their tissue4.
Furthermore, there are limitations with tissue biopsies, as they only provide a single snapshot of information of the tumor and do not account for the complexity of cancer and their evasive escape mechanisms. Tumors naturally evolve over time and can exhibit differences in PD-L1 expression longitudinally5. Tissue biopsies have several disadvantages, including limited accessibility to some tumors, especially inoperable ones, procedural side effects, including hemorrhage, infection, damage to nearby organs, or numbness around the biopsy site6, and high procedure-related costs7. Finally, there can be technical difficulties with tissue biopsies if the tissue amount is too low, not allowing molecular profiling to be completed to optimize regimens.


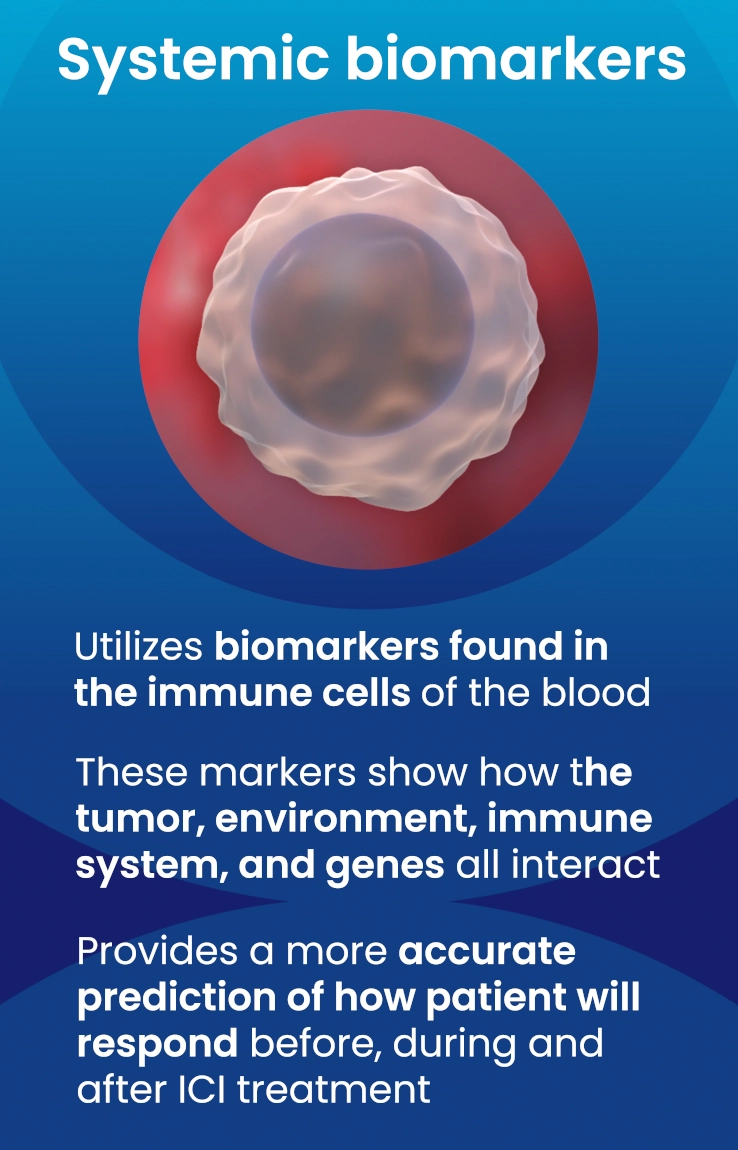
An alternative approach to address the shortcomings of tissue biopsy is liquid biopsies. Here, the samples analyzed are in liquid form, i.e., blood, urine, plasma, and other bodily fluids. The EpiSwitch® Checkpoint inhibitor Response-Test (CiRT), a first-of-its-kind blood test, is clinically available to determine a patient's response to ICIs with best-in-class accuracy. CiRT is a qPCR test that evaluates DNA regulatory (epigenetic) markers, called chromatin-conformational signatures, expressed in immune cells circulating the blood stream. Epigenomic configuration of the genome plays a pivotal role in regulating gene expression, and homeostasis of cellular phenotypes, including immune cells8. By analyzing the immune cells themselves, which is what the ICIs target specifically, CiRT can effectively assess the likelihood of response to this type of therapy.
EpiSwitch CiRT has many advantages over tissue biopsy analysis.
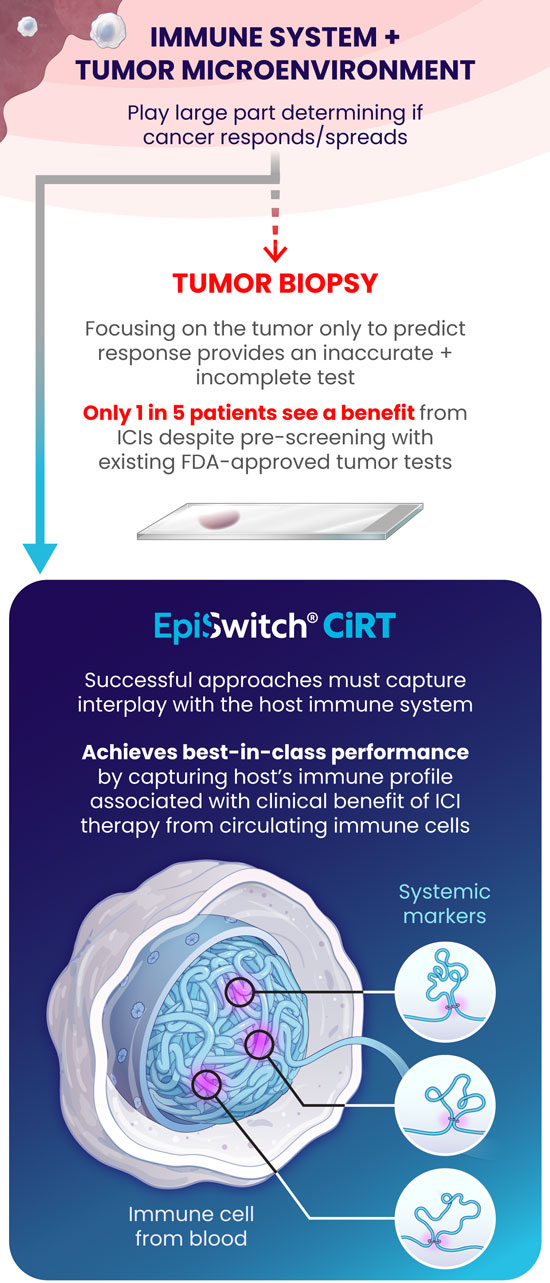
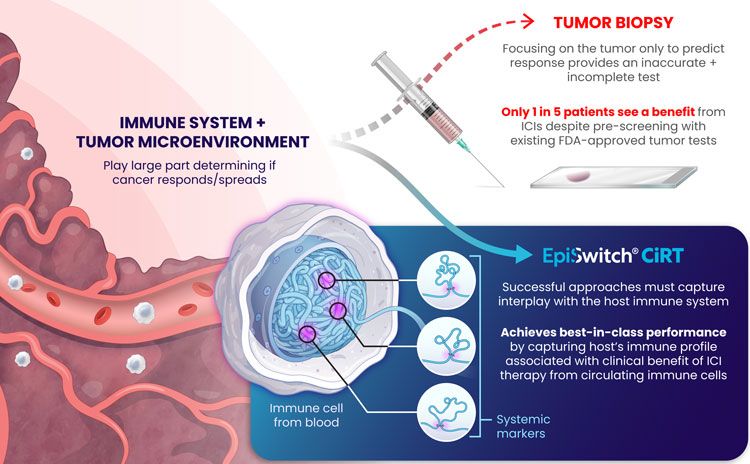
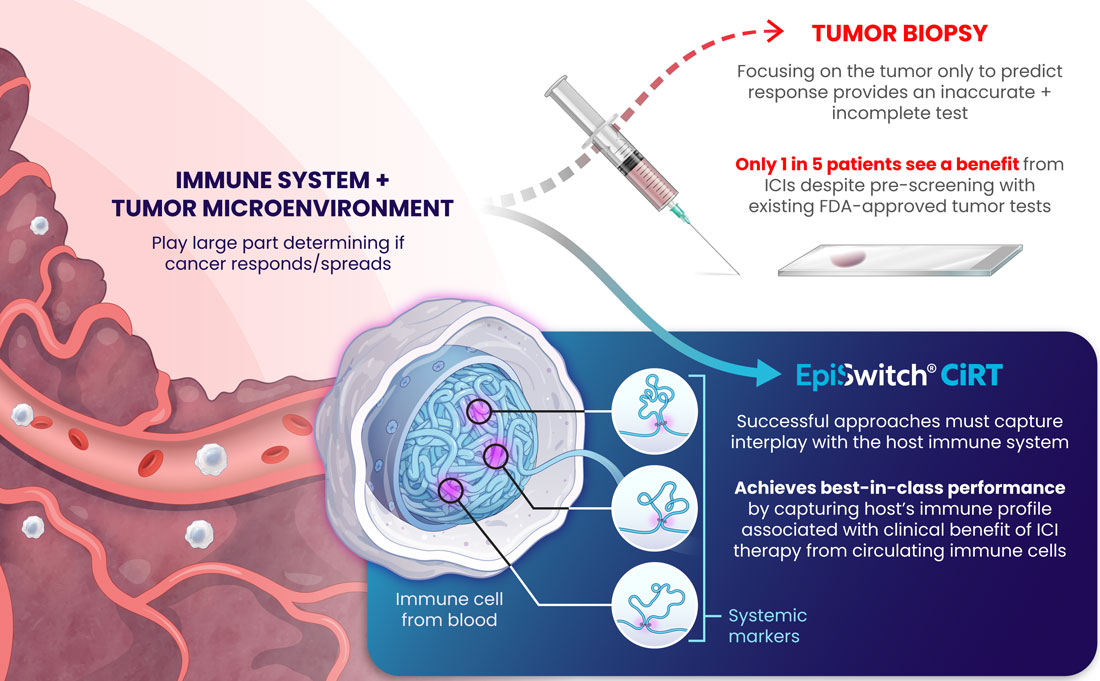
Altogether, CiRT is a great novel approach for oncologists to help fill the gaps existing within the current state of predicting response to ICI therapy, all without requiring tissue!
For more information, please visit: mycirt.com
References
1) Hoeben A, Joosten EAJ, van den Beuken-van Everdingen MHJ. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers (Basel). 2021;13(2):242. Published 2021 Jan 11. https://doi.org/10.3390/cancers13020242
2) Shiravand Y, Khodadadi F, Kashani SMA, et al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol. 2022;29(5):3044-3060. Published 2022 Apr 24. https://doi.org/10.3390/curroncol29050247
3) Kaira K, Imai H, Mouri A, Yamaguchi O, Kagamu H. Clinical Effectiveness of Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer with a Poor Performance Status. Medicina (Kaunas). 2021;57(11):1273. Published 2021 Nov 19. https://doi.org/10.3390/medicina57111273
4) Yang F, Wang JF, Wang Y, Liu B, Molina JR. Comparative Analysis of Predictive Biomarkers for PD-1/PD-L1 Inhibitors in Cancers: Developments and Challenges. Cancers (Basel). 2021;14(1):109. Published 2021 Dec 27. https://doi.org/10.3390/cancers14010109
5) Licata L, Mariani M, Rossari F, et al. Tissue- and liquid biopsy-based biomarkers for immunotherapy in breast cancer. Breast. 2023;69:330-341. https://doi.org/10.1016/j.breast.2023.03.014
6) Zhang Y, Shi L, Simoff MJ, J Wagner O, Lavin J. Biopsy frequency and complications among lung cancer patients in the United States. Lung Cancer Manag. 2020;9(4):LMT40. Published 2020 Aug 17. https://doi.org/10.2217/lmt-2020-0022
7) Kelly RJ, Turner R, Chen YW, Rigas JR, Fernandes AW, Karve S. Complications and Economic Burden Associated With Obtaining Tissue for Diagnosis and Molecular Analysis in Patients With Non-Small-Cell Lung Cancer in the United States. J Oncol Pract. 2019 Aug;15(8):e717-e727. https://doi.org/10.1200/JOP.18.00762 Epub 2019 Jun 25. PMID: 31237806.
8) Hunter E, Salter M, Powell R, et al. Development and Validation of Blood-Based Predictive Biomarkers for Response to PD-1/PD-L1 Checkpoint Inhibitors: Evidence of a Universal Systemic Core of 3D Immunogenetic Profiling across Multiple Oncological Indications. Cancers (Basel). 2023;15(10):2696. Published 2023 May 10. https://doi.org/10.3390/cancers15102696
9) Noor J, Chaudhry A, Noor R, Batool S. Advancements and Applications of Liquid Biopsies in Oncology: A Narrative Review. Cureus. 2023;15(7):e42731. Published 2023 Jul 31. https://doi.org/10.7759/cureus.42731
10) Yang, Y., Liu, H., Chen, Y. et al. Liquid biopsy on the horizon in immunotherapy of non-small cell lung cancer: current status, challenges, and perspectives. Cell Death Dis 14, 230 (2023). https://doi.org/10.1038/s41419-023-05757-5
Subscribe to receive our latest blog posts
Topics from groundbreaking discoveries to cutting-edge advancements and industry
trends.
A first-of-its-kind blood test to provide guidance on navigating the toughest challenges associated with use of an essential, widely-used class of cancer therapies: Immune Checkpoint Inhibitors.
Now every patient can benefit from the world’s first test to predict how a patient will respond to immune checkpoint inhibitor therapy with high accuracy.
Learn About CiRT